

MEDICAL DEVICE
DESIGN SERVICES
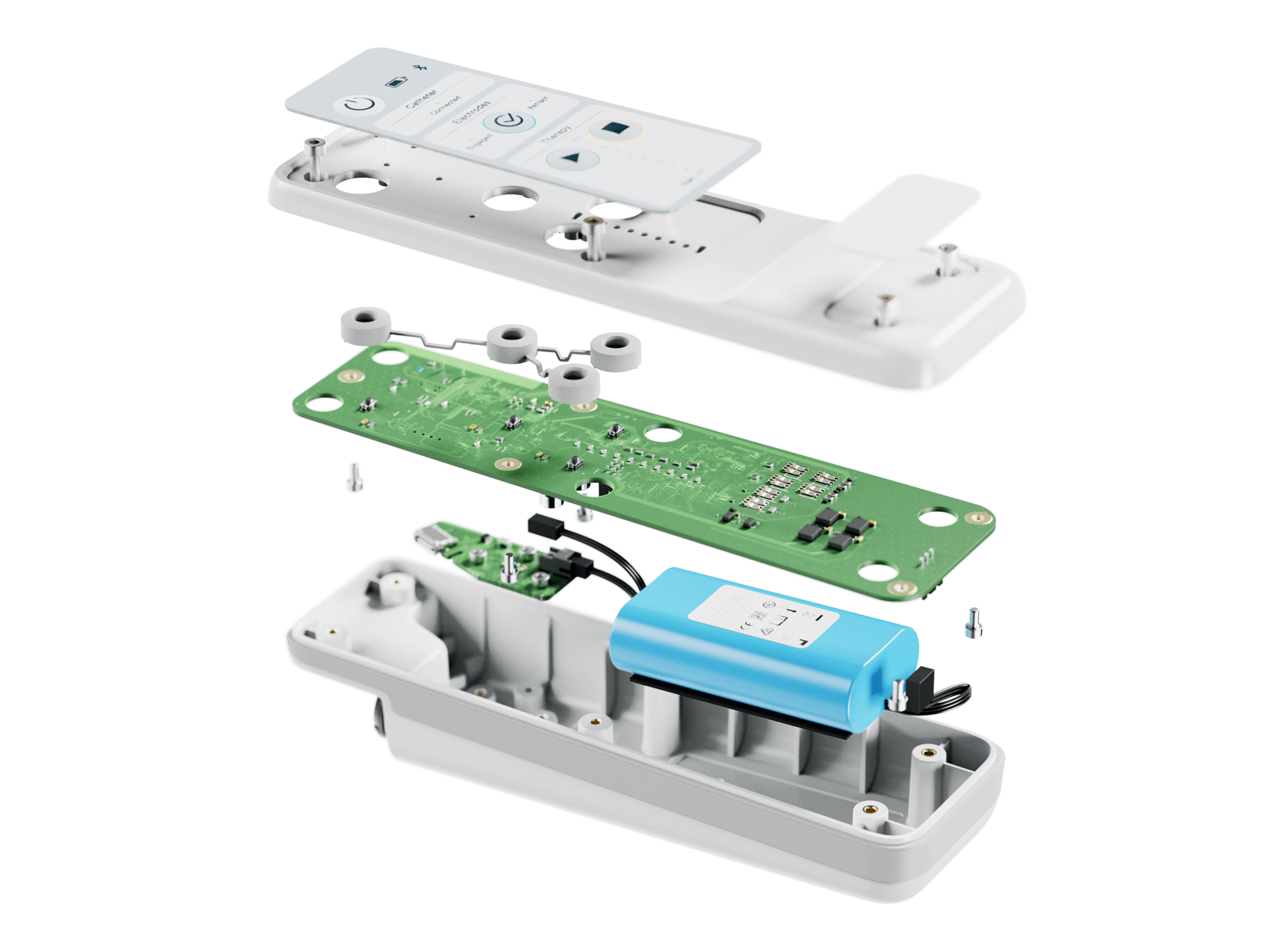
From early-stage R&D and design to prototyping and manufacturing, we here to help you get your medical product to market. Activities we undertake include:
- Medical technology R&D
- Electronic design
- Mechanical and industrial design
- Software development
- Smartphone Apps
- Cloud Servers and Portals
- Proof-of-Concepts
- Prototyping



Compliant with Industry Standards.
For new medical device startups, we can take a lean approach to medical device development. This is where regulatory requirements are considered up-front, but overheads are kept low until product design is matured and ready to undergo more restrictive design process controls.
For established medical device companies, we can work within your existing quality management system and certifications.
Boost Design can provide ISO13485 & FDA compliant design documentation, including:
- Design descriptions
- Design architecture documentation
- Risk analysis / DFMEAs (design failure mode effects analysis) to ISO14971
- SRS (system requirements specifications / traceability matrix)
- Verification plans, protocols and reports
- Design documentation for IEC62304 (medical device software)
- Design for IEC60601 (medical device essential safety and performance)
Designs and documentation may be included as a part of medical device regulatory submissions.
Your path
to success
With years of experience and a business mindset we have developed the Boost Method – a robust yet flexible process that regularly reviews a product’s viability and market fit.
We review the competitive landscape, market segments and target users, allowing our team to zero-in on a design strategy that positions your product to maximise commercial outcomes whilst minimising product development and manufacturing costs.
We understand the complexities and challenges that come with business, and we are focused on your success.

CASE STUDY
Thermal Armour
“The progress we have made over the last 6 months has been astounding. I couldn’t have done this without Boost… The design prototype they produced for military use was put together under incredible time restraints and despite being thrown out of a Globemaster (military plane), accidentally driven over by an Army jeep and left out in the rain overnight, the Army couldn’t break it. Considering it was a prototype, the quality of the work done by Boost was impressive…”
Nick Ralph
Director Thermal Armour
THE BOOST DIFFERENCE
Designing and developing any new device is tough. Designing and developing a medical device is even tougher, especially for new inventors and start-ups.
We’re well-versed in the complexities of medical device development and navigating the maze of stringent regulations. Our experienced team and vast network of expert partners can lead you on the journey.
From assessing efficacy with proof of concept testing to market demand analysis, patent development, and regulatory preparation, we tailor bespoke plans for seamless navigation through this sometimes complex landscape.
We’re masters of cutting-edge medical tech, especially with connected devices (such as the Internet of Medical Things – IoMT). This groundbreaking technology is reshaping healthcare, making remote healthcare services more accessible than ever.
IoMT devices, often used at point-of-care or home settings, collect data and send it to mobile phone apps, desktop apps, or the cloud. This simplifies remote monitoring, triggers automated alerts, and gathers vast datasets for better health insights and treatments.
These devices are the future of healthcare, and we’re experts in developing these technologies.
Regulations around medical devices are notoriously complex, and rightly so.
For start-ups we take a lean approach. We review regulatory considerations upfront to ensure designs are compliant, then keep documentation lean until product efficacy is proven. This provides a faster and more cost-efficient project.
For established medical device companies, we’ll work within your quality management system.
We can provide ISO13485 & FDA-compliant design documentation and work with a network of ISO13485-certified manufacturing partners to ensure that your product is made to the highest standards.
We’ve got you covered. With a full suite of product design and engineering services, we can take care of all aspects of your product development project, including software and hardware, from concept through to production.
Design and engineering is conducted in our well appointed Sydney HQ, ensuring that all aspects of your product design will integrate seamlessly together. Nothing falls through the cracks.
We’ll take care of the design and development of your Big Idea, so you can focus on spreading your vision and developing your business.












